iLink® Is the Only FDA-Approved Cross-Linking Procedure
The iLink cross-linking procedure with Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution), and the KXL® system is the first and only cross-linking procedure shown to be safe and effective by the FDA and it has been validated in clinical trials. The goal of the iLink® FDA-approved cross-linking procedure is to stiffen the cornea to slow or prevent further progression of keratoconus and preserve vision.
In the study that led to the FDA approval of iLink®, patients who did not undergo cross-linking continued to progress, while those who underwent the iLink® cross-linking procedure did not progress.[2],[3] It’s important to note that iLink® is not intended to improve vision, but rather to slow or halt the progression of the condition. Most patients will still need to wear corrective contact lenses or prescription eyeglasses following the procedure. Ideally, once progressive keratoconus is treated with iLink®, vision will most likely stabilize after a period of time; thereafter, it may be easier to fit patients in contact lenses and their prescription will not change as frequently.
Both the American Academy of Ophthalmology and the Cornea Society recommend cross-linking for the treatment of progressive keratoconus.[4] Specifically, the FDA-Approved iLink® cross-linking procedure is:
- Proven Safe & Effective: iLink® cross-linking is a minimally invasive treatment proven by the FDA to be safe and effective in slowing or halting the progression of keratoconus to preserve vision.
- FDA-Approved: iLink® is the first and only FDA-approved corneal cross-linking procedure and has been approved in the United States since April 2016.
- Validated in Clinical Trials: iLink® has been fully validated through randomized, controlled pivotal clinical trials that formed the basis of the FDA approval, demonstrating the efficacy and safety of the pharmaceutical eye drops, device, and procedure.
- Widely Covered by Insurance: iLink® cross-linking is widely covered by insurance and is the ONLY cross-linking procedure eligible for reimbursement in the U.S.
With any medical procedure, there are potential side effects. The most common ocular side effect is haze. Other ocular side effects include inflammation, fine white lines, dry eye, disruption of surface cells, eye pain, light sensitivity, reduced sharpness of vision, and blurred vision. The iLink® procedure is not right for everyone so talk to your physician to discuss what treatment option is right for you.
Only iLink® FDA-Approved Cross-Linking Is Eligible for Insurance Coverage
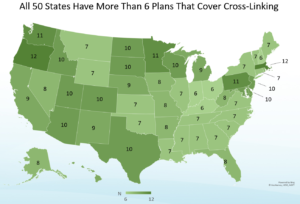
iLink® is widely covered by insurance and is the only cross-linking procedure that is eligible for insurance coverage in the United States. Specifically, more than 95% of the commercially insured population has access to FDA-approved cross-linking, and United Healthcare, Anthem, Aetna, Cigna & Humana are among the many commercial plans that cover iLink®. All 50 states have more than six insurance plans that cover the FDA-approved procedure.
For most insurance policies, iLink® is considered medically necessary for someone who has progressive keratoconus. It is also considered medically necessary for those with corneal ectasia following refractive surgery with documented worsening best spectacle-corrected visual acuity and irregular astigmatism. Insurance does not typically cover products and procedures that have not been approved by the FDA. For this reason, it is important to confirm with your physician that you are receiving iLink®.
If your insurance does not cover the procedure or if you are being asked to pay out of pocket, the cross-linking procedure you are being offered might not be approved by the FDA. For additional information on insurance coverage and to view the latest list of insurers known to have policies that cover the iLink®, visit the Insurance Information page.
Before undergoing cross-linking, make sure to do the following:
- Confirm with your doctor that he or she will be performing iLink® FDA-approved cross-linking with the Photrexa drug formulations and KXL System. You can also find a list of doctors performing iLink® on our physician locator.
- Contact your insurance provider to confirm they cover the iLink® FDA-approved cross-linking procedure. You can find a list of cross-linking insurance policies here.
If you do not have insurance and your doctor has recommended the iLink® FDA-approved cross-linking procedure, there are various programs available to help, such as the Glaukos Patient Assistance Program. This program is offered by individual doctors and practices, in partnership with Glaukos, to help cover the costs of the procedure and the Photrexa drug formulations if a patient does not have insurance coverage. Contact your doctor to learn more and to find out if you may qualify.
Make Sure You’re Receiving iLink® and Don’t Be Afraid to Get a Second Opinion!
You may want to seek a second opinion if your doctor recommends a cross-linking procedure that has not been FDA-approved. Here are some general guidelines to determine if you should seek a second opinion:
- Your doctor recommends a cross-linking procedure that has not been FDA-approved.
- Your doctor asks you to pay cash and submit to insurance on your own.
- Your doctor suggests that you pay for treatment as part of their own trial. (Clinical trials for FDA approval are typically free to patients).
- Your doctor tells you that their cross-linking procedure is not covered by insurance.
Remember: the only way they are performing FDA-approved cross-linking is if they are using Photrexa drug formulations and the KXL System. If you want to be prepared for your next appointment with your doctor, download this list of questions to ask your eye doctor about keratoconus and its treatments. Make sure you confirm that you are being offered FDA-approved cross-linking! If you are interested in learning more about iLink® and want to connect with a physician to discuss if the procedure is right for you, use our physician locator. To learn more about keratoconus and available treatment options, visit our website and follow Living with KC on Twitter, Facebook, and Instagram!
[1] U.S. Food & Drug Administration. (2017, January 17). Consumer Updates – Is It Really ‘FDA Approved?’ Retrieved from https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm047470.htm
[2] Data on File, Glaukos, Inc.
[3] Belin MW, Lim L, Rajpal RK, et al. Corneal cross-linking. Cornea. 2018;37(10):1218-1225.
[4] Garcia-Ferrer FJ, Akpek EK, Amescua G, et al. 2018 American Academy of Ophthalmology: Corneal Ectasia Preferred Practice Patterns: Ophthamol 2018;126(1)170-215